By Ncaba Ntshakala
The Ministry of Health has issued a nationwide recall of two batches of medication after discovering quality defects compromising their safety and efficacy.
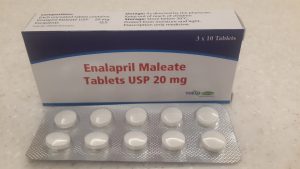
The affected drugs include Enalapril Maleate USP 20mg Tablets, Lot/Batch#: 136802, with an expiry date of March 2027, and Acetylsalicylic Acid BP 300mg Tablets, commonly known as Aspirin, under Lot/Batch#: ET240130, ET240131, ET240132, and ET240133, with an expiry date of May 2027.
The recall of Enalapril Maleate tablets stems from reports that tablets in blister packs disintegrate into small pieces and powder when some packs are opened.
Minister of Health Mduduzi Matsebula explained that broken pills pose a significant risk of underdosing, as portions of the tablet may be lost or not consumed, reducing the medication’s effectiveness.
RELATED: Eswatini on par with EU countries on free education and healthcare
This not only jeopardizes the safety of patients but also undermines the intended therapeutic outcomes.
For Acetylsalicylic Acid tablets, the recall was prompted by instances where the tablets were found sticking together, clumping, and forming a solid mass.
The Minister noted that such changes in form could arise from various factors, including improper handling during packing, transportation, or delivery.
These physical alterations render the medication unsuitable for consumption, as they compromise the drug’s usability and reliability.
The Ministry, through its Medicines Regulatory Unit (MRU), has initiated an extensive investigation to identify the root causes of these defects.
The goal is to understand how the changes occurred and implement measures to prevent similar incidents in the future.
All public health facilities across the country have been formally notified of the recall and instructed to facilitate the safe removal of these products from circulation.
Patients who have been prescribed these medications are advised to check the batch or lot numbers on their packages.
If their medication matches the affected batches, they should immediately inspect the tablets for signs of the defects described.
For Enalapril Maleate tablets, this includes disintegration into powder or broken pieces.
RELATED: Eswatini’s diabetes prevention efforts gain momentum – Minister of Health
For Acetylsalicylic Acid tablets, this involves checking for clumping or solid masses.
Patients are urged to cease consumption if these issues are observed and to return the medication to the nearest health facility for proper disposal. Replacements will be provided where necessary.
The Minister emphasized that the recall is a precautionary measure aimed at safeguarding public health and ensuring that all medications dispensed meet the highest standards of safety and efficacy.
Patients are encouraged to remain vigilant and report any irregularities with their medication to healthcare providers.


